Smart Food Safe participating in SQF Unites 2025, from March 2nd - 5th, 2025 at the Hyatt Regency, Orlando, Florida. Meet us at Booth #16 .
Smart Food Safe participating in SQF Unites 2025, from March 2nd - 5th, 2025 at the Hyatt Regency, Orlando, Florida. Meet us at Booth #16 .
Smart Food Safe participating in SQF Unites 2025, from March 2nd - 5th, 2025 at the Hyatt Regency, Orlando, Florida. Meet us at Booth #16 .
Smart Food Safe participating in SQF Unites 2025, from March 2nd - 5th, 2025
at the Hyatt Regency, Orlando, Florida. Meet us at Booth #16 .


Smart Food Safe participating in
Petfood Forum 2025, from April 28th to 30th in Kansas City, Missouri. Meet us at Booth #910.
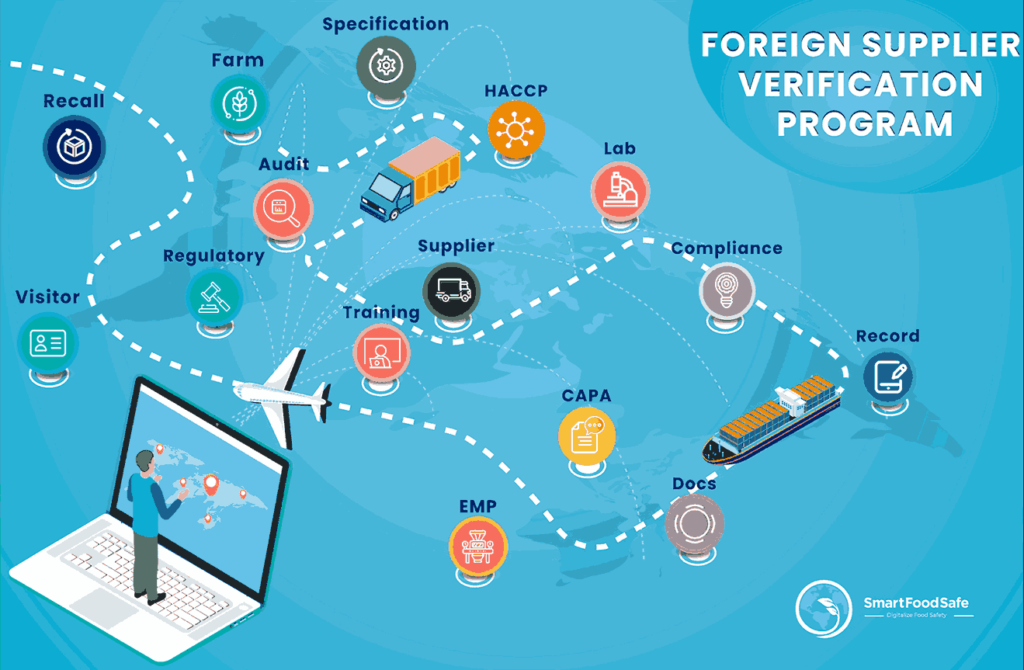
Supply Chain Management
A Comprehensive Guide To Streamline Compliance With Foreign Supplier Verification Program
A Comprehensive Guide To Streamline Compliance With Foreign Supplier Verification Program
Arundhathy Shabu
March 10, 2023

Supply Chain Management
A Comprehensive Guide To Streamline Compliance With Foreign Supplier Verification Program
Arundhathy Shabu
March 10, 2023
A Comprehensive Guide To Streamline Compliance With Foreign Supplier Verification Program
Supply Chain Management

Arundhathy Shabu
.
March 10, 2023
Did you know that between 2002 and 2019, a total of 22,459 pathogen/toxin violations were found in food imports from 110 different countries to the U.S., according to a 2021 study conducted by the USDA Economic Research Service?
However, this number only talks about import refusals due to microbial contamination based on a mere sampling, and the overall number of import refusals by the U.S. FDA will be much higher, considering the other causes of non-compliance. These statistics emphasize the necessity to enforce enhanced supervision to maintain global compliance for imported food items. This was why FDA introduced Foreign Supplier Verification Program (FSVP) on 16th November 2015 for Importers of Food for Humans and Animals, with an initial compliance date of 30th May 2017.
FDA released the initial draft guidance for FSVP requirements on 25th January 2018 that aimed to assist importers in building a successful FSVP. Retaining the core elements from the draft guidance and elucidating certain critical areas after observing the response from stakeholders, FDA released its final guidance on FSVP requirements on 11th January 2023.
Quick Recap on FSVP
The Food Safety Modernization Act (FSMA) came into force on 4th January 2011. It was a revolutionary step towards attaining enhanced food safety throughout the supply chain. According to FSMA, everyone who manufactures/processes, packages, or holds food must be responsible for food safety by complying with the FDA Food Safety Standards and Preventive Controls. FSMA requirements are applicable for domestic and foreign firms, and it was often challenging for FDA to inspect foreign enterprises to oversee compliance. As a solution, Congress came up with the Foreign Supplier Verification Program (FSVP).
FSVP is crucial to foreign suppliers exporting products to the U.S. and organizations in the U.S. importing products. The main objective of FSVP is to obligate U.S. food importers to verify that their foreign suppliers are manufacturing and processing food as per U.S. food safety requirements by adhering to safety regulations, preventive controls, and correct labeling to attain the optimum level of public health protection.
In a nutshell, FSVP is an important tool to protect the food supply system for consumers in assuring the safety of imported food by making importers liable to confirm that they only bring products into the U.S. that are held with the same safety standards as a domestically produced food item, along with complementing the existing import tools.
Key Elements Encompassed by FSVP
FSVP requires the U.S importers to develop, implement and maintain certain key elements of FSVP for each type of food they import. They are as follows:
Risk-Based Hazard Analysis
An importer must perform a written hazard analysis to identify naturally occurring, intentional, or unintentional biological, chemical, radiological, and physical food safety hazards. The hazards are detected based on the category of food imported, followed by an evaluation of the occurrence probability for the hazard and determination of the severity of illness if humans are exposed to the hazards. Importers can review and assess the hazard analysis process carried out by their foreign suppliers.
Country & Commodity Specific Risk Evaluation and Approval
FSVP importers are required to evaluate each foreign supplier’s performance based on the executed risk assessment and hazard analysis. They should evaluate the entity or entities that control the hazards, processes, and practices associated with ensuring food safety, compliance with applicable FDA regulations, and compliance history with food safety requirements. FDA mandates FSVP importers to only approve foreign suppliers who have been established to have satisfactory food safety compliance and proper control procedures for hazards. Further, the importers can only import from these ‘approved foreign suppliers.’
Supplier Verification Activities
For the approved foreign suppliers, FSVP importers must conduct verification processes to confirm that the hazards to be controlled are appropriately minimized or eliminated and that the suppliers maintain compliance. The importer can choose the type of verification activity and the frequency of performing the same depending on food risk and supplier evaluation. It is also imperative for importers to have written procedures that elaborate on those verification activities, which must be in place prior to the first importation event and systematically conducted subsequently. FDA’s Accredited Third-Party Certification Program recognizes “accreditation bodies” that will have the responsibility of accrediting third-party “certification bodies” that will conduct food safety audits and issue certifications for foreign food facilities. These audits will act as a verification tool and should adequately fulfill FSMA regulatory requirements for supplier verification.
Corrective Actions
Once the importer reviews the verification results and finds that the foreign supplier is producing food not in compliance or without meeting the necessary food safety requirements, they should take suitable corrective actions and document them. The corrective actions may include requiring the supplier to change the current procedures for compliance or food safety, adopting a different verification activity, or shifting to another foreign supplier.
Continuous Monitoring
The importers must continuously and regularly reevaluate the implemented monitoring processes to see if they maintain the prerequisites for FSVP. In the event of food safety incidents with imported food items or production site issues, or any other non-compliances, importers have to reevaluate the monitoring activities instantly.
Qualified Individual
A qualified individual pertains to FSVP and is defined by FDA’s Code of Federal Regulations Title 21 as “a person who has the necessary education, training, and experience (or a combination thereof) to perform an activity required under subpart C of this part, as appropriate to the individual’s assigned duties; this individual may be, but is not required to be, an employee of the establishment.” Only a qualified individual should carry out all FSVP-related activities.
FSVP Importer Identification
The importers should make sure that they are identified adequately with Importer Name, Email Address, and Unique Facility Identifier (UFI) as recognized by FDA at the time of the entry of the food product.
Record-Keeping
Documenting all the information concerning FSVP is vital to fully comply with the supplier verification regulations. The importer should maintain the records in the form of original records, true copies, or electronic records, which must be made available to the FDA upon request. All the records must be signed and kept up-to-date.
Clarity on Key Challenges Faced by FSVP Importers
Depending on the feedback received from stakeholders about the FSVP Draft Guidance, FDA made some modifications, including clarification on foods to which FSVP regulation is applicable, information to be contained in the FSVP, and who is required to implement and operate an FSVP. FDA also worked with Food Safety Preventive Controls Alliance to arrange training materials for FSVP importers. As a result, the final guidance for FSVP regulation came into existence.
The FSVP Final Guidance, provides recommendations for the industry in regard to performing risk-based foreign supplier verification activities for each food article they import into the United States to ascertain that they are produced in compliance with the applicable FDA regulations while not being adulterated or misbranded with respect to allergen labeling. Though the fundamental elements of the draft guidance are maintained, clarity is provided regarding the below points that the FDA had acknowledged as key challenges faced by importers to facilitate compliance with FSVP.
- FSVP ‘importer’ definition and scope of FDA’s FSVP:
For FSVP purposes, an ‘importer’ is defined as the individual or organization that is either the U.S. owner or consignee of the food being imported into the United States, and in cases when there are no such entities, the U.S. agency or representative of the foreign supplier is accepted as the importer at the time of entry, as confirmed in a signed statement of consent. If multiple entities meet the definition of “importer,” each must develop and maintain an FSVP for the imported food. The scope of FSVP majorly involves ensuring that these ‘importers’ perform risk-based activities for the imported foods, such as raw agricultural commodities, processed foods, dietary supplements, and animal feed, to meet U.S. safety standards and take corrective actions as required.
- FDA’s enforcement discretion policies:
FDA continues to exercise enforcement discretion for importers of food contact surfaces, certain grain raw agricultural commodities, and live animals imported for slaughter and processing at USDA-regulated establishments. Moreover, FDA also intends to extend enforcement discretion for importers whose foreign suppliers are subject to FDA enforcement discretion policy under its Produce Safety Rule or Preventive Controls for Human or Animal foods.
- FSVP compliance, approval, and verification requirements:
The final guidance elaborates on critical points regarding the FSVP design, evaluation, approval, and verification requirements. It included developing written FSVP verification procedures for each imported food, additional guidance on compliance assessment requirements for allergen misbranding and adulteration, FSVP mandates for a new facility or new food produced by the existing foreign supplier, etc.
- FSVP documentation and audit procedures:
FDA asserted that the importer must retain relevant documentation for each third-party audit. The documents should demonstrate that the importer verification activities yield sufficient assurance that the foreign supplier efficiently minimizes or prevents hazards requiring controls.
- Requirements regarding hazard analysis, hazard reports, and controls for identified hazards:
The final guidance introduced new conditions related to the discussion of hazard analyses, especially when an importer relies on a subsequent party in the distribution chain to control identified hazards. In such cases, the importers need to disclose that the food has not been processed to control the hazard but are not required to conduct an evaluation of the food or the foreign supplier. Instead, they have to obtain annual affirmation from the customer that the food will be processed in accordance with the food safety requirements and possess records of all versions of its hazard analysis, and document in written format if a known or foreseeable hazard in the food product requires control.
- FSVP requirements for importers of dietary supplements:
The final guidance contains additional information on importing dietary supplements. When neither the importer nor the supplier manufactures/processes the dietary supplement but only imports the dietary supplement components or dietary ingredients for manufacturing dietary supplements, then the importer must fully comply with the FSVP requirements as they do not qualify for the modified requirements for importers of foods subject to certain dietary supplement CGMP requirements.
Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.

Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.
Solutions to Overcome Challenges and Establish a Robust FSVP
The FSVP Final Guidance presents significant information regarding issues faced by FSVP importers. It is to be noted that those are suggestions that the importers can follow to achieve an ideal FSVP. Importers should also consider taking up solutions that can aid in streamlining their foreign supplier verification activities, such as
- Supplier Risk Assessment & Approval
- Paperless Audit Procedures
- Real-time Compliance Management
- Document Conformance System
- Risk-based Food Safety Plan
- Digital Record Management
It is high time for both importers and suppliers to comprehend and accept that the FSVP regulation can only be met expertly with accurate technological applications and systems in place, such as real-time assurance strategies, data-driven supply chain management, and other innovative digital solutions to help them adapt to the constantly shifting risk landscape in the food sector.
What SmartFoodSafe Offers to Achieve a Streamlined FSVP
SmartFoodSafe has become a one-stop solution for importers to incorporate various solutions for tackling different challenges and complying with FSVP regulations easily and cost-effectively.
Smart Supplier helps importers tackle the difficulty of carrying out the tedious supplier management process required in implementing FSVP through a risk-based supplier approval and performance management program.
Smart Audit enables importers and suppliers to perform risk-based verification effectively, focusing on providing assurance on controlling the enterprise’s top risks and improving compliance gaps through automated audit systems that replace conventional checklists with paperless auditing applications.
Smart Compliance can enhance the product verification compliance process with automated comparison against product specifications and improve the product release process.
Smart Doc facilitates food industries to be compliant with quality and food safety standards and stay updated with the latest program requirements through an integrated configurator for global standards and an electronic document management process.
Smart HACCP is a systematic preventive food safety tool that can help food businesses compose HACCP plans digitally for performing risk-based hazard analysis, identifying critical control points (CCP) or preventive control points (PCP), and controlling hazards.
Smart Record organizes the record management process by enabling paperless record-keeping to replace conventional paper records necessary to handle routine business operations in a digital and secure space.
Smart EMP transforms the evaluation process for pathogens, spoilage and indicator organisms, and allergens in a food facility by automating environmental monitoring and, thus, making it easy for foreign suppliers to demonstrate their production environment’s compliance with mandated hygiene and safety standards.
Smart Training can streamline employee training for industries digitally and will be useful in boosting employee knowledge on carrying out the different procedures of FSVP through the training materials provided by FDA for importers.
Our products provide supplier verification services that can be tailored to your business requirements while reducing costs and losses for both your enterprise as well as your suppliers to seamlessly develop and sustain a powerful FSVP.
FAQ
FSVP stands for Foreign Supplier Verification Program. It is a requirement under the U.S. FDA Food Safety Modernization Act (FSMA) that mandates importers to verify that foreign food suppliers meet U.S. food safety standards. It involves conducting risk assessments, verifying suppliers’ compliance, and maintaining proper documentation to ensure the safety of imported food.
FSVP (Foreign Supplier Verification Program) is a component of FSMA (Food Safety Modernization Act). FSVP focuses on verifying that imported food meets U.S. safety standards, requiring importers to conduct risk-based supplier evaluations, perform foreign supplier verification activities, and maintain appropriate documentation. FSMA is a comprehensive U.S. law that aims to prevent foodborne illnesses by shifting focus from response to prevention, implementing preventive controls, inspection and compliance requirements, and establishing regulations for food safety throughout the supply chain.
The process of country risk analysis involves assessing and evaluating the potential risks and opportunities associated with conducting business in a specific country. It includes analyzing political stability, economic factors, legal and regulatory frameworks, social and cultural aspects, and other relevant indicators to determine the level of risk and make informed decisions regarding investments or business operations.
The FSVP (Foreign Supplier Verification Program) Importer is the U.S. entity responsible for ensuring that imported food meets FDA safety standards. They are accountable for developing, implementing, and maintaining a FSVP, which includes verifying and documenting the safety practices of foreign food suppliers.
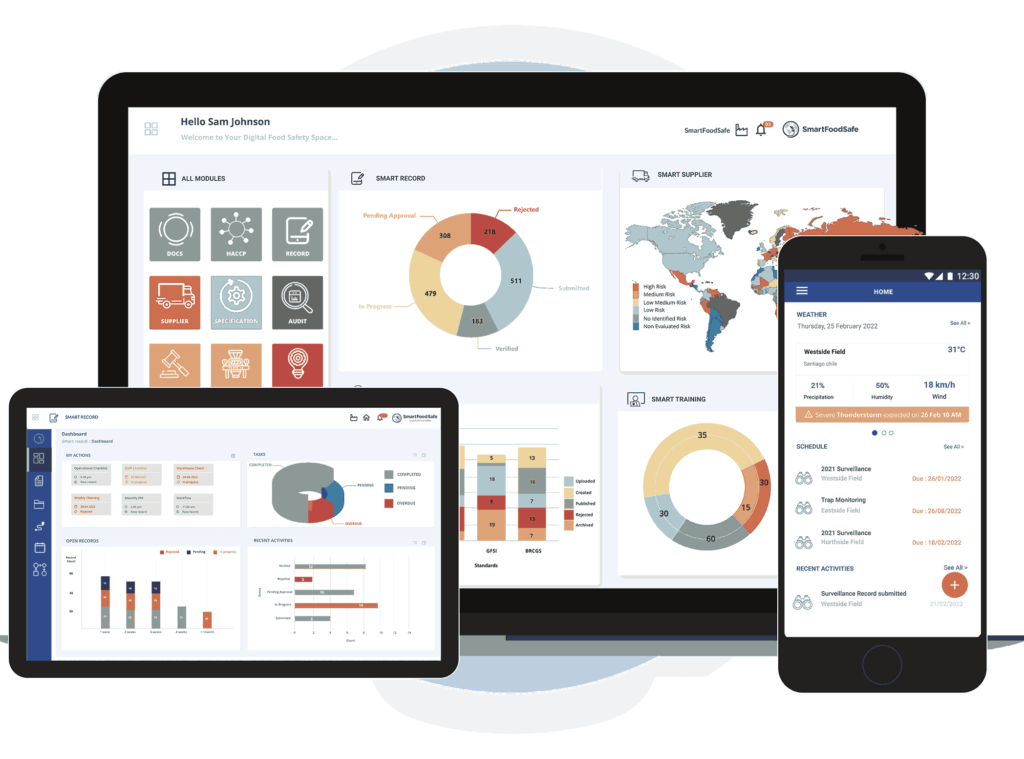
Quality and Food Safety Management Software
Food Safety and Quality Management Software to streamline processes, track compliance, ensure traceability and maintain audit readiness with global quality and food safety standards
Did you know that between 2002 and 2019, a total of 22,459 pathogen/toxin violations were found in food imports from 110 different countries to the U.S., according to a 2021 study conducted by the USDA Economic Research Service?
However, this number only talks about import refusals due to microbial contamination based on a mere sampling, and the overall number of import refusals by the U.S. FDA will be much higher, considering the other causes of non-compliance. These statistics emphasize the necessity to enforce enhanced supervision to maintain global compliance for imported food items. This was why FDA introduced Foreign Supplier Verification Program (FSVP) on 16th November 2015 for Importers of Food for Humans and Animals, with an initial compliance date of 30th May 2017.
FDA released the initial draft guidance for FSVP requirements on 25th January 2018 that aimed to assist importers in building a successful FSVP. Retaining the core elements from the draft guidance and elucidating certain critical areas after observing the response from stakeholders, FDA released its final guidance on FSVP requirements on 11th January 2023.
Quick Recap on FSVP
The Food Safety Modernization Act (FSMA) came into force on 4th January 2011. It was a revolutionary step towards attaining enhanced food safety throughout the supply chain. According to FSMA, everyone who manufactures/processes, packages, or holds food must be responsible for food safety by complying with the FDA Food Safety Standards and Preventive Controls. FSMA requirements are applicable for domestic and foreign firms, and it was often challenging for FDA to inspect foreign enterprises to oversee compliance. As a solution, Congress came up with the Foreign Supplier Verification Program (FSVP).
FSVP is crucial to foreign suppliers exporting products to the U.S. and organizations in the U.S. importing products. The main objective of FSVP is to obligate U.S. food importers to verify that their foreign suppliers are manufacturing and processing food as per U.S. food safety requirements by adhering to safety regulations, preventive controls, and correct labeling to attain the optimum level of public health protection.
In a nutshell, FSVP is an important tool to protect the food supply system for consumers in assuring the safety of imported food by making importers liable to confirm that they only bring products into the U.S. that are held with the same safety standards as a domestically produced food item, along with complementing the existing import tools.
Key Elements Encompassed by FSVP
FSVP requires the U.S importers to develop, implement and maintain certain key elements of FSVP for each type of food they import. They are as follows:
Risk-Based Hazard Analysis
An importer must perform a written hazard analysis to identify naturally occurring, intentional, or unintentional biological, chemical, radiological, and physical food safety hazards. The hazards are detected based on the category of food imported, followed by an evaluation of the occurrence probability for the hazard and determination of the severity of illness if humans are exposed to the hazards. Importers can review and assess the hazard analysis process carried out by their foreign suppliers.
Country & Commodity Specific Risk Evaluation and Approval
FSVP importers are required to evaluate each foreign supplier’s performance based on the executed risk assessment and hazard analysis. They should evaluate the entity or entities that control the hazards, processes, and practices associated with ensuring food safety, compliance with applicable FDA regulations, and compliance history with food safety requirements. FDA mandates FSVP importers to only approve foreign suppliers who have been established to have satisfactory food safety compliance and proper control procedures for hazards. Further, the importers can only import from these ‘approved foreign suppliers.’
Supplier Verification Activities
For the approved foreign suppliers, FSVP importers must conduct verification processes to confirm that the hazards to be controlled are appropriately minimized or eliminated and that the suppliers maintain compliance. The importer can choose the type of verification activity and the frequency of performing the same depending on food risk and supplier evaluation. It is also imperative for importers to have written procedures that elaborate on those verification activities, which must be in place prior to the first importation event and systematically conducted subsequently. FDA’s Accredited Third-Party Certification Program recognizes “accreditation bodies” that will have the responsibility of accrediting third-party “certification bodies” that will conduct food safety audits and issue certifications for foreign food facilities. These audits will act as a verification tool and should adequately fulfill FSMA regulatory requirements for supplier verification.
Corrective Actions
Once the importer reviews the verification results and finds that the foreign supplier is producing food not in compliance or without meeting the necessary food safety requirements, they should take suitable corrective actions and document them. The corrective actions may include requiring the supplier to change the current procedures for compliance or food safety, adopting a different verification activity, or shifting to another foreign supplier.
Continuous Monitoring
The importers must continuously and regularly reevaluate the implemented monitoring processes to see if they maintain the prerequisites for FSVP. In the event of food safety incidents with imported food items or production site issues, or any other non-compliances, importers have to reevaluate the monitoring activities instantly.
Qualified Individual
A qualified individual pertains to FSVP and is defined by FDA’s Code of Federal Regulations Title 21 as “a person who has the necessary education, training, and experience (or a combination thereof) to perform an activity required under subpart C of this part, as appropriate to the individual’s assigned duties; this individual may be, but is not required to be, an employee of the establishment.” Only a qualified individual should carry out all FSVP-related activities.
FSVP Importer Identification
The importers should make sure that they are identified adequately with Importer Name, Email Address, and Unique Facility Identifier (UFI) as recognized by FDA at the time of the entry of the food product.
Record-Keeping
Documenting all the information concerning FSVP is vital to fully comply with the supplier verification regulations. The importer should maintain the records in the form of original records, true copies, or electronic records, which must be made available to the FDA upon request. All the records must be signed and kept up-to-date.
Clarity on Key Challenges Faced by FSVP Importers
Depending on the feedback received from stakeholders about the FSVP Draft Guidance, FDA made some modifications, including clarification on foods to which FSVP regulation is applicable, information to be contained in the FSVP, and who is required to implement and operate an FSVP. FDA also worked with Food Safety Preventive Controls Alliance to arrange training materials for FSVP importers. As a result, the final guidance for FSVP regulation came into existence.
The FSVP Final Guidance, provides recommendations for the industry in regard to performing risk-based foreign supplier verification activities for each food article they import into the United States to ascertain that they are produced in compliance with the applicable FDA regulations while not being adulterated or misbranded with respect to allergen labeling. Though the fundamental elements of the draft guidance are maintained, clarity is provided regarding the below points that the FDA had acknowledged as key challenges faced by importers to facilitate compliance with FSVP.
- FSVP ‘importer’ definition and scope of FDA’s FSVP:
For FSVP purposes, an ‘importer’ is defined as the individual or organization that is either the U.S. owner or consignee of the food being imported into the United States, and in cases when there are no such entities, the U.S. agency or representative of the foreign supplier is accepted as the importer at the time of entry, as confirmed in a signed statement of consent. If multiple entities meet the definition of “importer,” each must develop and maintain an FSVP for the imported food. The scope of FSVP majorly involves ensuring that these ‘importers’ perform risk-based activities for the imported foods, such as raw agricultural commodities, processed foods, dietary supplements, and animal feed, to meet U.S. safety standards and take corrective actions as required.
- FDA’s enforcement discretion policies:
FDA continues to exercise enforcement discretion for importers of food contact surfaces, certain grain raw agricultural commodities, and live animals imported for slaughter and processing at USDA-regulated establishments. Moreover, FDA also intends to extend enforcement discretion for importers whose foreign suppliers are subject to FDA enforcement discretion policy under its Produce Safety Rule or Preventive Controls for Human or Animal foods.
- FSVP compliance, approval, and verification requirements:
The final guidance elaborates on critical points regarding the FSVP design, evaluation, approval, and verification requirements. It included developing written FSVP verification procedures for each imported food, additional guidance on compliance assessment requirements for allergen misbranding and adulteration, FSVP mandates for a new facility or new food produced by the existing foreign supplier, etc.
- FSVP documentation and audit procedures:
FDA asserted that the importer must retain relevant documentation for each third-party audit. The documents should demonstrate that the importer verification activities yield sufficient assurance that the foreign supplier efficiently minimizes or prevents hazards requiring controls.
- Requirements regarding hazard analysis, hazard reports, and controls for identified hazards:
The final guidance introduced new conditions related to the discussion of hazard analyses, especially when an importer relies on a subsequent party in the distribution chain to control identified hazards. In such cases, the importers need to disclose that the food has not been processed to control the hazard but are not required to conduct an evaluation of the food or the foreign supplier. Instead, they have to obtain annual affirmation from the customer that the food will be processed in accordance with the food safety requirements and possess records of all versions of its hazard analysis, and document in written format if a known or foreseeable hazard in the food product requires control.
- FSVP requirements for importers of dietary supplements:
The final guidance contains additional information on importing dietary supplements. When neither the importer nor the supplier manufactures/processes the dietary supplement but only imports the dietary supplement components or dietary ingredients for manufacturing dietary supplements, then the importer must fully comply with the FSVP requirements as they do not qualify for the modified requirements for importers of foods subject to certain dietary supplement CGMP requirements.
Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.

Solutions to Overcome Challenges and Establish a Robust FSVP
The FSVP Final Guidance presents significant information regarding issues faced by FSVP importers. It is to be noted that those are suggestions that the importers can follow to achieve an ideal FSVP. Importers should also consider taking up solutions that can aid in streamlining their foreign supplier verification activities, such as
- Supplier Risk Assessment & Approval
- Paperless Audit Procedures
- Real-time Compliance Management
- Document Conformance System
- Risk-based Food Safety Plan
- Digital Record Management
It is high time for both importers and suppliers to comprehend and accept that the FSVP regulation can only be met expertly with accurate technological applications and systems in place, such as real-time assurance strategies, data-driven supply chain management, and other innovative digital solutions to help them adapt to the constantly shifting risk landscape in the food sector.
What SmartFoodSafe Offers to Achieve a Streamlined FSVP
SmartFoodSafe has become a one-stop solution for importers to incorporate various solutions for tackling different challenges and complying with FSVP regulations easily and cost-effectively.
Smart Supplier helps importers tackle the difficulty of carrying out the tedious supplier management process required in implementing FSVP through a risk-based supplier approval and performance management program.
Smart Audit enables importers and suppliers to perform risk-based verification effectively, focusing on providing assurance on controlling the enterprise’s top risks and improving compliance gaps through automated audit systems that replace conventional checklists with paperless auditing applications.
Smart Compliance can enhance the product verification compliance process with automated comparison against product specifications and improve the product release process.
Smart Doc facilitates food industries to be compliant with quality and food safety standards and stay updated with the latest program requirements through an integrated configurator for global standards and an electronic document management process.
Smart HACCP is a systematic preventive food safety tool that can help food businesses compose HACCP plans digitally for performing risk-based hazard analysis, identifying critical control points (CCP) or preventive control points (PCP), and controlling hazards.
Smart Record organizes the record management process by enabling paperless record-keeping to replace conventional paper records necessary to handle routine business operations in a digital and secure space.
Smart EMP transforms the evaluation process for pathogens, spoilage and indicator organisms, and allergens in a food facility by automating environmental monitoring and, thus, making it easy for foreign suppliers to demonstrate their production environment’s compliance with mandated hygiene and safety standards.
Smart Training can streamline employee training for industries digitally and will be useful in boosting employee knowledge on carrying out the different procedures of FSVP through the training materials provided by FDA for importers.
Our products provide supplier verification services that can be tailored to your business requirements while reducing costs and losses for both your enterprise as well as your suppliers to seamlessly develop and sustain a powerful FSVP.
FAQ
FSVP stands for Foreign Supplier Verification Program. It is a requirement under the U.S. FDA Food Safety Modernization Act (FSMA) that mandates importers to verify that foreign food suppliers meet U.S. food safety standards. It involves conducting risk assessments, verifying suppliers’ compliance, and maintaining proper documentation to ensure the safety of imported food.
FSVP (Foreign Supplier Verification Program) is a component of FSMA (Food Safety Modernization Act). FSVP focuses on verifying that imported food meets U.S. safety standards, requiring importers to conduct risk-based supplier evaluations, perform foreign supplier verification activities, and maintain appropriate documentation. FSMA is a comprehensive U.S. law that aims to prevent foodborne illnesses by shifting focus from response to prevention, implementing preventive controls, inspection and compliance requirements, and establishing regulations for food safety throughout the supply chain.
The process of country risk analysis involves assessing and evaluating the potential risks and opportunities associated with conducting business in a specific country. It includes analyzing political stability, economic factors, legal and regulatory frameworks, social and cultural aspects, and other relevant indicators to determine the level of risk and make informed decisions regarding investments or business operations.
The FSVP (Foreign Supplier Verification Program) Importer is the U.S. entity responsible for ensuring that imported food meets FDA safety standards. They are accountable for developing, implementing, and maintaining a FSVP, which includes verifying and documenting the safety practices of foreign food suppliers.

Quality and Food Safety Management Software
Food Safety and Quality Management Software to streamline processes, track compliance, ensure traceability and maintain audit readiness with global quality and food safety standards


