 English
▾
English
▾
An important aspect that becomes the decisive factor for consumers in picking a food item off the shelf is its ‘best before’ or ‘use by’ date, but have you ever wondered how that period is determined? Here’s where the concept of ‘shelf life study’ comes to the forefront.
In the realm of food production and distribution, a shelf life study is a key tool in achieving the safety, quality, and longevity of food products, and delving into the nuances of this essential process provides a roadmap for identifying the product’s durability guaranteeing its wholesomeness and nutritional integrity until the end of its shelf life.
What is Meant By Shelf Life?
Shelf life refers to the duration, under specific storage conditions, within which food remains safe, maintains its desired taste, chemical composition, and physical attributes, and meets label specifications.
For foods that are stable in terms of microbes, like those kept at room temperature or frozen, oxidative reactions often dictate the end of their shelf life. Quality is a constantly changing state, gradually declining, so the shelf life of these products hinges on how quickly oxidation occurs and reaches an unacceptable level. This ‘acceptability limit’ denotes the point at which the food becomes unfit for consumption.
Shelf life holds various meanings: to consumers, it’s the timeframe indicated on the best-before label, signifying how long they can keep the food before disposing of it. It indicates when the food is no longer suitable for consumption. Yet, shelf life can also denote the duration a food item can be stored and exhibited while still retaining acceptable quality or specific functions.
Passing the shelf life date doesn’t instantly render a product unsafe; rather, it means it no longer meets predefined quality standards. Some well-preserved products, like pasteurized milk, can stay fresh beyond their shelf life if stored properly and uncontaminated by bacteria. However, for certain items where bacterial growth can occur, consuming them past their shelf life could pose health risks, leading to foodborne illnesses. Such products usually have set shelf life and expiry dates to align.
Factors Impacting Shelf Life
Conducting shelf life studies encompasses a holistic assessment, taking into consideration the intrinsic (related to the product) and extrinsic (external) conditions that influence a product’s shelf life. Understanding these intrinsic and extrinsic properties aids in assessing the precise point at which a product surpasses its optimal state.
Intrinsic Factors
- Ingredient Quality: The quality, consistency, and storage of ingredients significantly influence the final product. Microbiological variations in ingredients can impact safety and shelf life.
- Formulation/Composition: The combination of ingredients determines the growth of spoilage organisms. Changes in proportions or ingredients can affect shelf life; some microorganisms can outgrow pathogens, spoiling the product before it becomes unsafe.
- Water Activity (aw): The amount of water available to microorganisms is critical. Certain ingredients and processing methods affect aw, influencing pathogen growth and product quality.
- pH Level: Acidity or alkalinity influences microorganism growth. Most pathogens are inhibited below a pH of 4.6, while spoilage organisms like yeasts and molds are acid-tolerant.
- Preservatives: Additives control microorganism growth, extending shelf life. For instance, nitrates in meat products inhibit spoilage.
Extrinsic Factors
- Processing: Methods like high-pressure processing affect shelf life, increasing durability, and safety.
- Oxygen Availability: Altering air around food via vacuum or modified atmosphere packaging extends shelf life by limiting microbial growth.
- Packaging Materials: Packaging safeguards food from contamination and can determine expected shelf life, relying on specific characteristics like light barriers.
- Storage Conditions: Temperature, humidity, light exposure, and protection from contaminants impact microbial growth. Refrigeration slows some microorganisms but not all pathogens.
- Distribution Chain: Handling and storage during distribution affect shelf life, especially considering temperature fluctuations.
- Consumer Practices: While outside a manufacturer’s control, consumer practices impact shelf life. Instructions for proper storage and handling of labels are crucial.
Comprehending and managing these components for deciphering the shelf life assists in examining and maintaining the intended taste and quality of the food, preservation of its microbial, chemical, and physical properties, and sustaining its freshness and adherence to nutritional specifications.
Understanding Shelf Life Studies
Shelf life studies evaluate how a food product reacts to both intrinsic and extrinsic factors. They serve to provide documented proof required for demonstrating to both retailers and regulators that the food maintains freshness, taste, nutritional value, and quality until the expiration of the “best before” date on the unopened package, given proper storage conditions. This information also guides manufacturers to comply with food safety and quality standards efficiently, reducing waste, and enhancing consumer trust.
Types of Shelf Life Studies
The following approaches are frequently employed to conduct a food shelf life assessment:
1. Direct or Real-time Study
In a real-time shelf life study, food is stored under standard conditions for a duration surpassing the estimated shelf life. Regular evaluations ascertain the food’s condition, identifying the point when it loses its expected quality, nutritional value, and physical integrity.
2. Indirect or Accelerated Shelf Life Study
In an accelerated shelf life study, predictive techniques using factors like increased temperatures hasten the food’s deterioration process to estimate its shelf life more rapidly. Data gathered from accelerated deterioration measurements, such as microbial content, can be used in mathematical models to predict spoilage rates and bacterial growth under typical conditions.
Accelerated studies are often preferred for foods with extended shelf lives. However, a comprehensive understanding of the food’s formulation and properties is necessary to accurately interpret the data.
Validation is crucial for ensuring the appropriateness and effectiveness of indirect studies in predicting shelf life. Concurrent real-time and indirect studies, known as dual studies, aid in validating the anticipated shelf life.
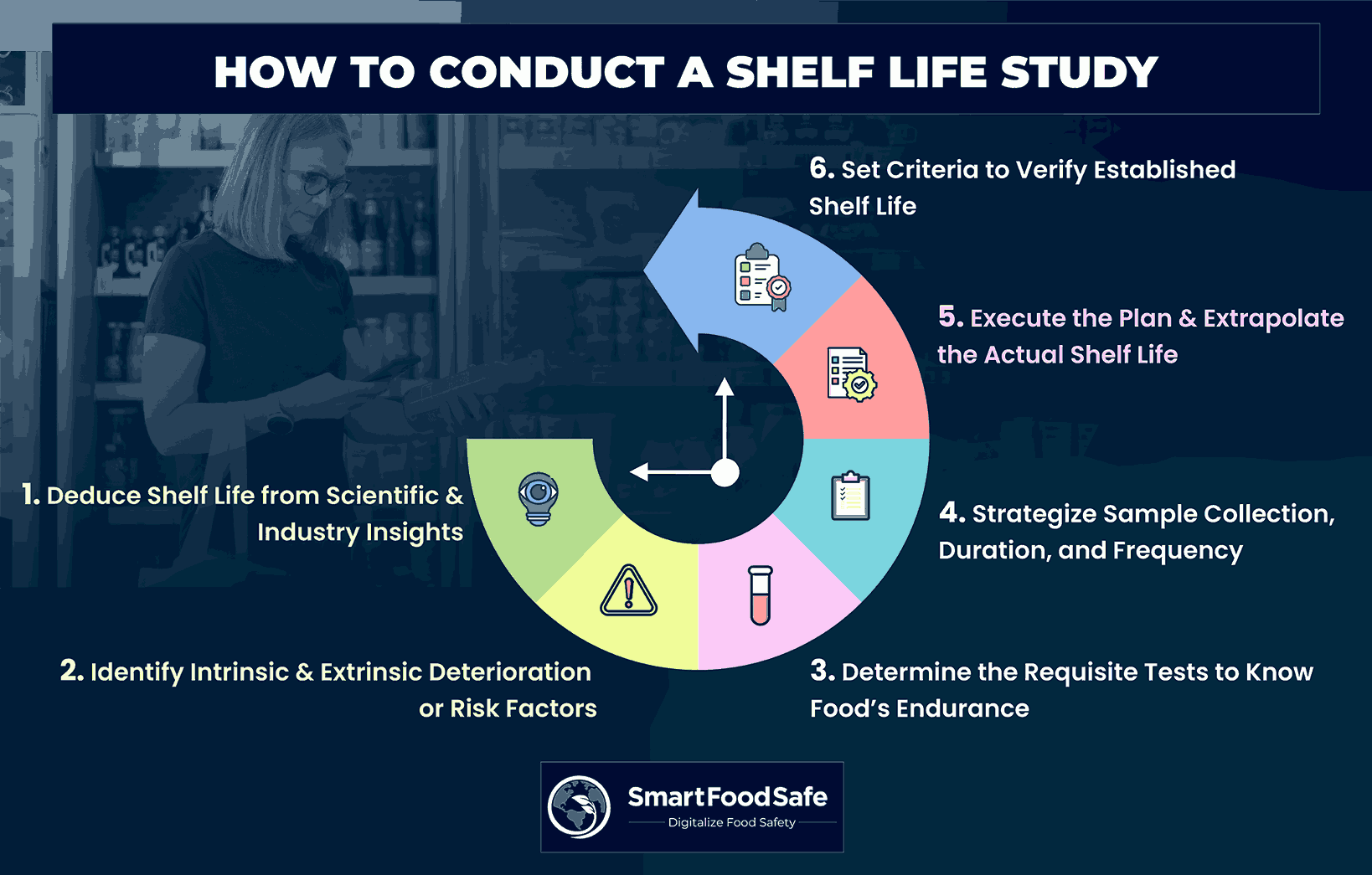
Steps Involved in Carrying Out a Shelf Life Study
Step 1: Assess the Shelf Life of the Food
Examine data from scientific literature, industry resources, and various publications. This includes analyzing historical data related to any past illnesses, outbreaks, or potential microbial growth linked to the specific type of food product.
Utilize the gathered information to suggest an initial shelf life duration, serving as a foundational step in establishing the actual shelf life of the product.
Step 2: Recognize Factors Leading to Food Deterioration or Risk
During this stage, you pinpoint the characteristics influencing the food’s shelf life. This data serves as a guide for conducting tests that evaluate these traits, aiding in pinpointing the stage at which the food becomes unsafe for consumption.
Various properties, both inherent and external, play a role in determining the shelf life of different food products. These properties, whether intrinsic or extrinsic, serve as Critical control points affecting shelf-life duration.
Step 3: Determine the Requisite Tests to Quantify the Food’s Endurance
For the shelf life study, it is vital to identify tests that verify the food’s safety, confirming its desirable sensory, microbiological, chemical, and physical attributes, and ensuring compliance with any nutritional or label declarations and are adapted to the unique qualities of the particular food product being evaluated.
Step 4: Plan the Shelf Life Study With Estimated Shelf Life as the Initial Point
Strategizing a shelf life study demands meticulous planning, necessitating thorough exploration of existing literature concerning relevant shelf life studies conducted on your particular food product. This process involves the following elements:
- Selection of Tests: The choice of tests depends on the specific food item being assessed and should at least adhere to relevant food safety/quality standards. Seeking guidance from credible third-party entities, such as laboratories or food process authorities, for suitable tests and associated criteria is advisable.
- Sample Collection: Samples for shelf life studies are derived from the same production run after final packaging. Emphasis on testing finished products that represent the least favorable scenario within established process limits ensures representative test outcomes.
- Duration of Study: For direct shelf life studies, the initial testing period aligns with the estimated “best before” date. Continuation of the study occurs if qualifying criteria are met post the estimated period, terminated otherwise. Moreover, adjustment of product formulation may be required to achieve the desired shelf life, with a subsequent decrease in testing duration for establishing a shorter shelf life.
- Sampling Frequency: Short-lived perishable products (7 to 10 days) typically demand daily sampling and testing. Longer shelf life products require sampling at the beginning, midpoint, end of estimated shelf life, and at least one point beyond.
- Sample Quantity: At least duplicate containers should be tested at each sampling interval to account for variability. Discard samples after testing; increased samples at intervals enhance confidence in study validity.
- Storage Conditions: During the study, store product samples under the least favorable conditions anticipated for their shelf life duration. Regularly monitor and record temperature and humidity levels in storage.
Step 5: Execute the Planned Steps & Extrapolate the Actual Shelf Life
Document your plan and record all the activities conducted and the results obtained. At some juncture during the sampling and testing phase of the shelf life study, the food might cease to meet safety, nutritional, or quality benchmarks. Assess the compiled data to ascertain the duration for which the food can be stored while sustaining safety, nutritional value, and acceptable quality.
Reliable interpretation of gathered data relies on a well-designed and executed analysis, such as the use of appropriate microbiological standards.
The food’s shelf life is established based on the shortest duration before it fails any quality or safety criterion. This actual shelf life denotes the point at which the product no longer satisfies quality and/or safety requirements.
The stated “best before” date on the product label should ideally reflect the confirmed shelf life, incorporating a safety margin (for instance, the actual shelf life reduced by one or more days for safety purposes).
Step 6: Set Criteria to Verify Established Shelf Life
Ensuring ongoing adherence to safety and quality standards throughout the declared shelf life is significant. Conduct shelf life tests biannually or triannually, depending on the food’s shelf life, to consistently collect evidence supporting the established shelf life’s validity. This procedure involves sampling from various distribution and retail points, and testing key factors like acidity, flavor, and spoilage, identified as pivotal in the shelf life study.
Repeat the shelf life study following any alterations in formulation, ingredient quality, packaging, production methods, processing equipment, or sanitation. Adjust the declared shelf life if testing indicates its inappropriateness, or if customer complaints regarding food quality emerge before the “best before” label date.
Optimizing shelf study processes in the food industry is paramount and recent trends emphasize the need for faster, more accurate assessments to meet evolving consumer preferences, regulatory standards, and sustainability demands. The incorporation of automation and digitalization in shelf-life study procedures such as Laboratory Information Management Systems (LIMS) can be the game changer integral for streamlining and elevating the efficiency of these steps. These technological advancements not only improve productivity but also enable businesses to respond swiftly to market changes, reduce costs, and ultimately deliver fresher, safer products to consumers.
Enhance Your Shelf Life Study Process with Smart Food Safe
Smart Food Safe‘s comprehensive platform allows for meticulous specification management through Smart Specification , enabling teams to outline intricate details, track alterations, and collaborate seamlessly to bring product ideas to fruition.
It also accommodates Smart Lab, a powerful and versatile Laboratory Information Management Solution (LIMS) that empowers laboratories to effectively manage processes from sample reception to final analysis, with well-integrated features for supervising and automating the workflow for conducting an expedited shelf life study.
Moreover, Smart Lab also equips laboratories to prepare quotes, onboard customers, register samples, integrate instruments, update and validate results, generate certificates, and create invoices and reporting, enabling industrial laboratories to adequately manage resources, improve performance, and ensure data integrity.
This integration allows enterprises to not only conduct in-depth shelf life studies but to efficiently oversee the entire product lifecycle. From designing and testing to production and distribution, this solution brings uninterrupted coordination across departments. Hence, Smart Food Safe presents an amalgamation of these innovative solutions that not only elevates laboratory efficiency but also drives the success and reliability of products within the global food industry.

 French
French
 Spanish
Spanish
 Portuguese
Portuguese
