[yoobar_scode id =" 40763" title = "Banner"]
[yoobar_scode id =" 40788" title = "Mobile banner"]


Food Safety Management
Essential ingredients to Enhance the Safety of Pet Food Manufacturing
Essential ingredients to Enhance the Safety of Pet Food Manufacturing
Arundhathy Shabu
Oct 10, 2023

Food Safety Management
Essential ingredients to Enhance the Safety of Pet Food Manufacturing
Arundhathy Shabu
Oct 10, 2023
Essential ingredients to Enhance the Safety of Pet Food Manufacturing
Food Safety Management

Arundhathy Shabu
.
Oct 10, 2023
A food safety incident that occurred in December 2020, causing the deaths of hundreds of pets due to contaminated pet food, sent shockwaves across the pet food industry and pet owners all over the globe. It resulted in the recall of about 60 million pounds of corn-based dog and cat food containing deadly levels of aflatoxins, despite the FDA’s maximum permissible limit of aflatoxin in pet food to be 20 parts per billion.
These unfortunate deaths could have been prevented if efficient safety management systems had been in place to control the food safety risks associated with the ingredients of pet foods.
All ingredients and processes used in the creation of pet food and treats are potential hazards, as rightly said by Gary Huddleston, Director of Feed Manufacturing and Regulatory Affairs, American Feed Industry Association (AFIA), in the article published in the July 2023 issue of Pet Food Processing. This is why pet food manufacturers need to appropriately monitor and regulate all elements of the ingredient life cycle to safeguard the safety of their pet products.
What is Pet Food?
Any product produced by a pet food manufacturer, whether processed, partially processed, or unprocessed, intended to be ingested by pet animals after being placed on the market. It is any commercial product formulated to provide essential nutrients and energy for domesticated animals and to meet their dietary requirements, considering factors like age, size, breed, and activity level. Pet food typically includes a combination of proteins, carbohydrates, fats, vitamins, and minerals, all tailored to support the pet’s health and growth.
Pet food is more than just a meal; it is a source of nutrition and sustenance for pet animals. To maintain proper bodily functions and ensure a pet’s well-being, it is crucial to be mindful of the ingredients in their food as well as ascertain optimal levels of safety management throughout the pet food production and processing till they reach the pets.
Why is it Important & Challenging to Ensure Food Safety in Pet Foods?
Ensuring food safety in pet foods is crucial for several reasons, as it comes with unique challenges such as:
Lack of Communication: Unlike humans, pets cannot verbally express their feelings or discomfort when they experience food-related problems. This makes it tedious to identify and trace back the source of foodborne illnesses or issues. In the case of human food safety incidents, if someone falls ill, they can describe what they ate, where they ate it, and when they consumed it. In contrast, pets cannot provide this information, making it difficult to pinpoint the exact cause of their illness.
Inconsistent Dietary Habits: Pets often have varied and unpredictable eating habits. They might graze throughout the day, eat from different sources, or consume various types of foods. This inconsistency in their dietary patterns further complicates the process of identifying the source of foodborne illnesses.
Limited Awareness and Understanding: Animals lack awareness and understanding of what they are eating. They do not have the ability to make choices based on food safety and quality considerations. This means that even if a pet food product is contaminated or of low quality, pets will not be able to avoid it. They rely on their owners or caregivers to make safe and healthy food choices for them.
Delayed Symptoms: In some cases, symptoms of foodborne diseases in pets may not appear immediately. It might take days or even weeks for signs of illness to manifest. This delayed onset of symptoms can make it even more problematic to link the illness to a specific food source.
Understanding Pet Food Ingredients
Pet food ingredients can be derived from either animals or plants. In a study conducted in 2020, it was discovered that pet food recipes utilize over 500 different ingredients, amounting to a total of more than 8.5 million tons of both animal- and plant-based products.
Many animal-derived ingredients used in pet food come from parts of the animal that are not consumed by humans due to cultural preferences and eating habits. These parts, such as lungs, hearts, kidneys, or liver, are perfectly safe for consumption once they have undergone veterinary inspections and been deemed suitable to consume.
On the other hand, ingredients of plant origin are often shared between human and pet nutrition, including items like maize, rice, wheat, and oats. Some ingredients are unique to pet food, as manufacturers seek particular combinations of nutritional properties, particularly beneficial for pets. These may include vegetable fibers to promote healthy digestion and pre-and probiotics to support digestive health, among others.
Maintaining Ingredient Safety by Controlling Hazards in Pet Foods
Each ingredient included in a pet food recipe and listed on the label should be carefully chosen by pet food manufacturers to serve a function for the pet. There are various types of hazards that can be present in pet food ingredients, according to the FDA’s Guidance for Industry, “Hazard Analysis and Risk-Based Preventive Controls For Animals,” each of which should be thoroughly supervised.
Known or Reasonably Foreseeable Hazards
Pet food can become contaminated with biological, chemical (including radiological), or physical hazards, depending on the type of pet food being manufactured, processed, packed, or held at your facility.
- Biological Hazards
Prominent biological hazards are bacterial pathogens (Salmonella spp., L. monocytogenes, and pathogenic E. coli), viral pathogens, and certain parasites (Toxoplasma gondii, Cryptosporidium spp.) that may contaminate pet food manufacturing operations. These pathogens can be:
i) Ingredient-related hazards – i.e., introduced from raw materials and other ingredients
ii) Process-related hazards – e.g., if the pathogens:
- survive the manufacturing process
- increase in number due to lack of time/temperature control or due to the animal food’s formulation
- are introduced into a finished animal food due to loss of container integrity
iii) Facility-related hazards – e.g., if the pathogens are introduced from:
- unsanitary animal food processing equipment
- cross-contamination between raw and cooked products
- contaminated air
- sewage or contaminated water
- Chemical Hazards
Chemical hazards include that are natural components of ingredients (e.g., glucosinolates) or natural toxins (e.g., mycotoxins), contaminants of raw materials and other ingredients (e.g., pesticides and drug residues), and hazards as a result of manufacturing errors (e.g., nutrient deficiencies or toxicities). These chemical hazards can be:
i) Ingredient-related hazards – i.e., introduced from raw materials and other ingredients such as:
- pesticide residues and mycotoxins on raw agricultural
- commodities and grains
- heavy metals in or on raw agricultural commodities or in mineral
- ingredients or premixes
- natural toxins (e.g., glucosinolates in the Brassicaceae family)
- animal drug residues
- unapproved food or color additives
- dioxins
ii) Process-related hazards – e.g., from manufacturing errors or cross-contamination such as:
- nutrient deficiencies or toxicities due to manufacturing error
- animal drug carryover from medicated to non-medicated animal
- food
- food or color additives not approved for certain species due to
- incomplete cleanout of equipment
iii) Facility-related hazards – e.g., from chemicals used on animal food processing equipment or utensils or chemicals stored in the facility such as:
- contamination with industrial chemicals such as cleaners or sanitizers
- chemicals not used in processing animal food but stored in a facility like fertilizers
- heavy metals due to leaching from containers or utensils
- Physical Hazards
Physical hazards are broadly classified as sharp hazards, choking hazards, and conditions of
animal food hazards such as size and hardness. These include:
- Metal (Ferrous and Non-Ferrous): Metal-to-metal contact during processing can introduce metal fragments into products. For example, metal fragments can break off during mechanical cutting and blending operations, and some metal equipment has parts that can break or fall off, such as wire-mesh belts. Metal screens may become worn over time or be torn, introducing metal fragments
- Glass: Glass fragments in animal food can cause injury to the animal eating the food. Most animal food facilities do not use glass containers for their animal food, but fragments may be introduced through the environment (e.g., overhead light fixtures made of glass that can fracture) or through ingredients that are contaminated with glass
- Hard Plastic: Hard plastic can be introduced into animal food when tools and equipment such as scoops, paddles, buckets, or other containers develop fatigue, crack, and break as they wear. It can also be introduced into animal food when plastic sieves and screens deteriorate.
- Conditions of Animal Food: It refers to the physical, mechanical, and other characteristics (e.g., particle size, hardness, surface roughness, digestibility, and ability to soften when moistened) of animal food that can cause injuries or illness in animals. Hazards related to the conditions of the animal food can occur when the particle size is too large to eat, resulting in starvation (e.g., crumbles too large for small birds) or alternatively, when the food is ground too fine as it can aerosolize and cause respiratory problems and corneal injuries. Also, a large portion of fines (i.e., very small particles from the milling or pelleting process) can lead to rapid fermentation by gut microflora, resulting in bloat in ruminants (e.g., cattle). Lack of digestibility can result in an obstructed digestive tract.
- Radiological Hazards
Contamination of pet food by radionuclides (a radiological hazard) is a rare event. The most common way these radionuclides are incorporated into animal food is through the use of water that contains the radionuclides. This water may be an ingredient in animal food or used during the manufacturing process, such as for washing ingredients or equipment. They may also result from accidental contamination, e.g., contamination arising from accidental release from a nuclear facility or from damage to a nuclear facility from a natural disaster.
Securing Safety Measures Throughout Ingredient Lifecycle: The Cornerstone for Safe Pet Foods
The pet food industry is witnessing a substantial transformation driven by several key factors. First, the increasing pet population has expanded the market for pet food, with more households adopting pets. Concurrently, pet owners are changing their preferences, seeking high-quality, safe, and natural ingredients for their furry family members. There’s a growing emphasis on pet health and wellness, driving the demand for pet food that contributes to overall well-being and supports specific health needs.
Furthermore, businesses that can provide sustainable, tailored, and transparent pet food options are well-positioned in this advancing landscape. Customized nutrition based on factors like age, breed, and health condition is gaining popularity, and pet owners increasingly value ingredient traceability and ethical sourcing. Social media also plays a pivotal role in influencing pet owner preferences and trends, making it essential for businesses to engage with pet owners through these platforms, showcase their commitment to quality and sustainability, and stay responsive to changing demands. This evolving market offers significant opportunities for companies that can align with these pet owner preferences and the broader shift towards more responsible and individualized pet food choices.
An overview of the key aspects of securing pet food safety in the journey of ingredients to your pet’s bowl in reference to the FEDIAF Guide To Good Practice for the Manufacture of Safe Pet Foods and GAPFA Global Pet Food Safety Guidance is given below.
Pet Food Safety Management System
The national and regional pet food industry associations and companies of pet food manufacturers across the globe establish their own guidelines to set up pet food safety management systems based on internationally recognized standards, e.g., EN-ISO 9000:2005 series, ISO 22000:2005 (or FSSC 22000), PAS 222, and Global Food Safety Initiative benchmark requirements.
A pet food safety management system should encompass several key components. It must establish a documented system that outlines the policy objectives of the pet food establishment, ensuring the production of safe and suitable pet food products. It should describe the organizational structure, defining roles and responsibilities related to product safety, as well as the allocation of resources and personnel training.
The system should include procedures for all stages of production, storage, and distribution while relying on records to validate and verify the safety and quality of pet food products. There should be detailed operational hygiene and process controls to maintain a clean and safe production environment. Furthermore, it should incorporate a Food Safety Risk Assessment plan for each product family produced, such as HACCP or HARPC along with internal audits and management reviews, with documented results and corresponding preventive/corrective actions. Finally, it must include a system for handling raw materials and packaging materials, including traceability to ensure product quality and safety. This system should be reviewed regularly for continuous improvement on at least an annual basis or if there is any change to the food safety system.
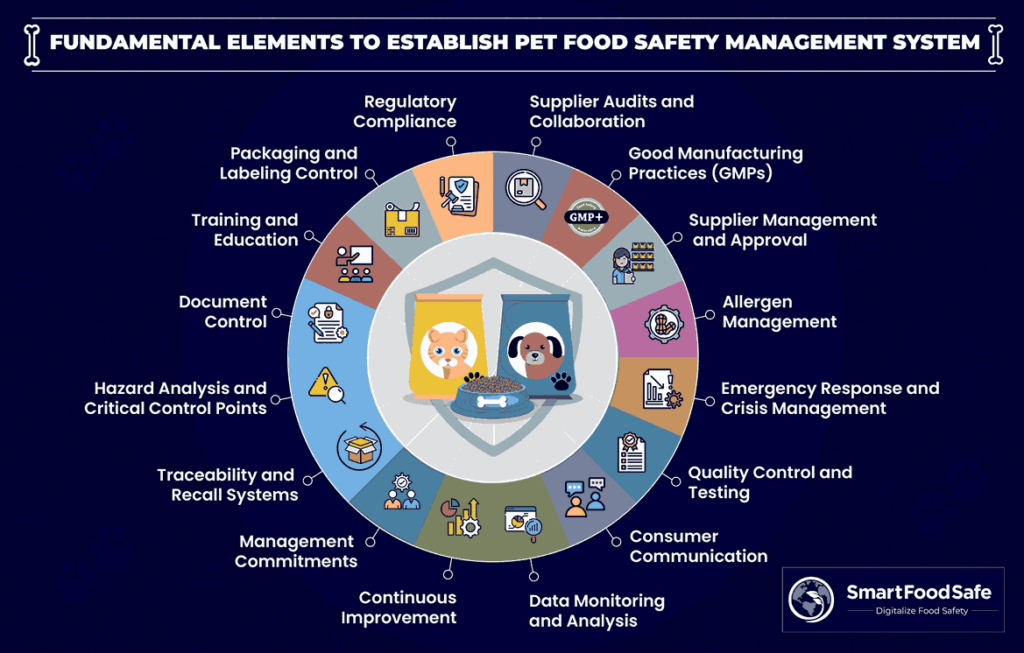
Let us delve into the fundamental elements of establishing a pet food safety management system:
1. Management Commitment
The management, spanning from higher to lower levels, must be dedicated to implementing pet food safety practices, including establishing a safety policy, ensuring regulatory compliance and good manufacturing practices, defining the scope of the safety management system, identifying potential risks to pet food production, and having a crisis management plan in place. Management-appointed staff should have clear responsibilities and authority to identify and document safety issues and initiate preventive and corrective actions to address product safety concerns.
1.1 Management Responsibility
The senior site management of a pet manufacturing facility mandates the creation and communication of a food safety policy, the establishment of a food safety culture, and the implementation of a food safety management system that complies with regulations and customer requirements. They must lead in setting food safety objectives, allocating resources, and fostering accountability among staff. It also necessitates the designation of responsible individuals, including backup personnel, for various roles within the food safety management system. Adequate staffing and alignment of departments to meet food safety goals are emphasized. Training and competencies of site personnel are to be ascertained, and the integrity of the food safety system must be maintained during organizational or personnel changes.
1.2 Management Review
The pet food safety management system requires an annual review by management, including updates to system documentation, assessment of food safety culture performance, evaluation of food safety objectives and performance measures, analysis of corrective and preventative actions, trends from audits, customer complaints, and verification activities, examination of the hazard and risk management system, and the review of follow-up actions from previous management assessments. Detailed records of all management reviews and updates must be retained for documentation purposes.
1.3 Complaint Management
The procedure for addressing food safety complaints related to pet food produced or handled on-site, consisting of the documentation and implementation of a process to handle complaints from commercial customers, consumers, and authorities, must be in place. Adverse trends in customer complaints must be investigated, with a focus on determining the root causes by knowledgeable personnel. Appropriate corrective and preventative actions should then be taken based on the severity of the incident and the root cause analysis. Records of customer complaints, their investigation, and the resolution process must be maintained for documentation and accountability purposes.
2. Pre-requisite Programmes
The following prerequisite programs outline the general pet food safety principles across the food supply chain:
2.1 Facilities & Plant Design
The site must be located and maintained to prevent contamination and should have measures in place to ensure ongoing effectiveness. External storage should protect items from contamination, and drainage should prevent standing water. The surrounding areas should be clean, well-kept, and pest-controlled. Waste collection areas should be clearly defined and managed to prevent contamination. Access to the site needs to be controlled to address security concerns.
Internally, the construction of the site should suit its purpose. The production process should allow for proper cleaning and disinfection to prevent contamination and cross-contamination. Sufficient working and storage space is needed. Segregation of materials and waste is essential to minimize cross-contamination, and facilities for disposing of unused animal byproducts should be available.
In terms of construction, walls, floors, and ceilings should facilitate cleaning and maintenance. Floors should have proper drainage. Ceilings and fixtures should prevent dirt, condensation, mold, and dust accumulation. Windows for ventilation should be screened to prevent pests. External openings should be managed to prevent foreign matter, moisture, and pests. Ample ventilation is required in product storage and processing areas. Finally, personnel hygiene facilities should be available and clearly designated, separate from production areas.
2.2 Utilities
The design and layout of utilities must take into account the prevention of product contamination. Whenever applicable, utility units must be placed away from walls to allow easy access for operation, cleaning, and maintenance and to prevent pest infestation. Utilities include:
Supply of water or steam as product ingredient or for product contact;
Supply of air (or gas) as ventilation (natural or mechanical) or as a compressed air or gas system;
Supply of energy (for example, electricity, light, or heat);
2.3 Waste Disposal
Effective waste management is important to prevent contamination in production areas. Waste collection, removal, and disposal systems should be in place. Waste containers must be clearly marked, positioned in designated areas, designed for easy cleaning and emptying, used exclusively for waste, and kept closed. Drains should be constructed and located to prevent material or product contamination, with adequate capacity to handle expected flow loads. Drainage should not carry contaminants from dirty to clean areas. Maintaining cleanliness and controlling spoilage and dust is essential to deter pests.
2.4 Equipment
Equipment in pet food production should be designed and placed to prevent contamination and enable effective cleaning and disinfection. Machinery that contacts feed materials or pet food should be dried after wet cleaning. Proper specification and commissioning of equipment, along with regular maintenance following the manufacturer’s procedures, are mandatory to maintain equipment. All equipment surfaces in contact with the product should be non-reactive and impermeable. Equipment used to monitor critical control points for Product Safety, Operational Prerequisite Programs, and product legality should be calibrated and traceable.
2.5 Cleaning & Sanitation
Maintaining hygienic conditions in pet food production requires establishing standards of hygiene and housekeeping. Only approved feed-grade cleaning and sanitizing agents should be used. Cleaning tools and equipment must be well-maintained to prevent potential sources of contamination. Cleaning and disinfection programs should be documented and cover the entire facility and equipment. These programs must be validated and verified for their effectiveness in reducing contamination risk, with periodic cleaning and sanitizing activities recorded.
2.6 Personnel
Personnel personal hygiene policies must be established, documented, and enforced. Staff involved in various aspects of production should be trained on these policies and other relevant procedures for product safety. The policies should cover hand washing, rules for eating, drinking, and smoking, clothing requirements, jewelry and watch usage, treatment of injuries, and staff access to post-kill-step product areas. Staff should flow between different areas to prevent cross-contamination, and facilities should be kept clean. Storage for personal effects and protective clothing, where necessary, should be provided, with separate eating and smoking areas away from production facilities. Visitors and contractors at the plant should adhere to the same personal hygiene procedures.
Training requirements for all staff, including temporary personnel and contractors, should be identified and documented. This includes induction training specific to the facility, covering product safety, personal hygiene, and relevant prerequisites like GMPs. Specific training needs, such as in food safety risk assessment systems (e.g., HACCP), and internal auditing, should be identified and scheduled. Records of training activities, competency assessments, and certificates should be maintained. The training program’s effectiveness should be periodically assessed.
2.7 Integrated Pest Management System
The pet food manufacturer is responsible for implementing procedures to minimize the risk of pest infestation, including hygiene, cleaning, materials inspection, and storage monitoring. Pest control programs should be in place and regularly reviewed for effectiveness. The manufacturer can either contract a competent and licensed pest control organization or have trained personnel for premises inspection and treatment. Electric fly killers and pest traps, if used, should be positioned to avoid product contamination. Detailed documentation on bait use and pest control measures’ locations on a site plan is necessary, along with keeping records of inspections, recommendations, and actions taken to address pest issues.
2.8 Foreign Bodies Control Policy
The use of glass or other brittle material (such as hard plastic components in equipment) close to production machinery must be avoided, and wherever necessary, it must be protected against breakage. Implement a zoning plan as identified in hazard assessments. Have written procedures in place for handling wood, metal, glass, and hard clear plastic breakages in various areas of the production process to ensure necessary precautions. These procedures should be part of a formal foreign body control policy. Establish measures to prevent, control, or detect potential contaminants identified in hazard assessments.
3. HACCP System
The team responsible for a pet food facility should establish a system that assesses potential food-related hazards in the manufacturing, processing, packaging, or storage of pet food. This system should identify and put into practice preventive measures to significantly reduce or eliminate these hazards. It is essential to continually monitor the effectiveness of these controls and maintain routine records of this monitoring and validation.
The applied Food Safety Risk Assessment system, such as HACCP, HARPC, or an equivalent, should adhere to internationally accepted standards. To conduct a food safety risk assessment, it should follow the steps and principles outlined in the ‘Hazard Analysis and Critical Control Point (HACCP) system and guidelines for its application’ as detailed in the Annex to Codex Alimentarius ‘Recommended International Code of Practice – General Principles of Food Hygiene’ (CAC/RCP 1).
The HACCP system is carried out in 12 steps, which follow the seven principles described in the Codex Alimentarius based on a prior implementation of pre-requisite programs.
Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.

Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.
| Principle | Description | Steps |
| Codex Principle 1 | Conduct a hazard analysis | Step 1: Assemble HACCP Team Step 2: Describe product Step 3: Identify intended use Step 4: Construct flow diagram Step 5: Confirm flow diagram on site Step 6: List all potential hazards Step 6: Conduct a hazard analysis Step 6: Consider control measures |
| Codex Principle 2 | Determine the Critical Control Points (CCPs) and Operational Pre- Requisite Programmes (OPRPs) | Step 7: Use decision tree to determine CCPs and OPRP |
| Codex Principle 3 | Establish critical limits | Step 8: Establish action and critical limits for each CCP and OPRP |
| Codex Principle 4 | Establish CCP monitoring procedures | Step 9: Establish monitoring systems for each CCP and OPRP |
| Codex Principle 5 | Establish corrective action plans | Step 10: Establish the corrective action to be taken when monitoring indicates that a particular CCP or OPRP is not under control |
| Codex Principle 6 | Establish verification procedures | Step 11: Establish procedures for verification to confirm that the HACCP system is working effectively |
| Codex Principle 7 | Establish documentation and record keeping systems | Step 12: Establish documentation concerning all procedures and records appropriate to these principles and their application |
4. Production Practices in Pet Manufacturing
The production practices in pet manufacturing should be such that the manufacturing environment and personnel should not be a source of risk to product safety.
4.1 Management of Incoming Materials
Incoming feed materials, additives, and packaging materials must meet safety and legal standards. Procedures should cover registration, inspection, analysis, acceptance, and rejection of these materials, including how to handle and track rejected deliveries.
Vendor/Supplier Approval & Assessment System: A system for approving vendors and suppliers is paramount for sourcing safe feed materials. It includes site approval, purchase control, and documentation of standards and monitoring procedures for primary production, inbound materials, and packaging. Approved supplier lists should be maintained, and assessment may involve continuous monitoring, inspection, traceability, HACCP system evaluation, product safety, and legislative compliance.
Specifications: Written specifications must define safety and legal compliance for feed materials, additives, and packaging materials and be regularly updated. Each material should have a specification agreed upon with the relevant parties. Specifications reflect risk analysis for physical, chemical, and biological risks per HACCP. Procedures should exist for specification amendment and approval across the process.
Handling of Incoming Materials: Inspect vehicles, documentation, and materials before unloading to confirm correctness and suitability. Check incoming materials against specifications using Certificates of Analysis (COA). Document procedures for inspection, sampling, and analysis, including information on products, hazards, methods, frequency, qualifications, and responsibilities. Establish procedures for dealing with non-conforming materials and document rejected shipments. Maintain documentation of rejected shipments, including reasons for rejection. Ensure perishable or frozen materials meet minimum temperature requirements during shipment, transportation, and receipt, and document temperature checks at receiving points for perishable goods.
4.2 Pathogens Monitoring
When a pet food safety risk linked to pathogens is defined in the HACCP study, a monitoring program should be implemented in order to proactively control the safety of products. Pathogen monitoring is a way to ensure the safety of pet food and is one of the programs aimed at measuring the effectiveness of the implementation of the pre-requisite programs, in particular for products that are not sterilized in hermetic packages. Zoning and a pre-defined flow of products and personnel is an important prerequisite program to proactively prevent pathogen presence.
In order to monitor the microbiological state of a factory, samples are collected and analyzed according to a pre-defined plan (location, frequency, number). Data obtained from the monitoring should be routinely trended, and the information obtained will drive subsequent sampling and product release protocols as well as corrective actions. The type of pathogen (and indicator) microorganisms to be monitored will be defined by the hazard analysis (HACCP plan). Pathogen monitoring control programs must be implemented, including environmental samples, line samples, and finished product samples, and should be subject to regular review.
4.3 Transport & Storage
All vehicles and warehouses used for storing or transporting ingredients and products must be suitable for their intended purpose and maintained in a hygienic condition. Ingredient usage and shipping schedules should follow a FIFO or FEFO strategy where possible. Storage areas should be designed to prevent damage, contamination, mixing, or deterioration. Clear identification of storage areas is necessary, and regular inspections for structural integrity and content conditions, especially in dry ingredients, are vital. Adequate ventilation and capacity for chilled and frozen products are required. Storage areas should be kept clean and free from contamination risks. Procedures to prevent cross-contamination of finished products, packaging, and ingredients should be in place, and processed pet food and packaging material must be separated from unprocessed ingredients to avoid cross-contamination.
4.4 Mixing & Homogeneity
Mixers used in pet food manufacturing should be suitable for the range of weights or volumes they handle and capable of producing homogeneous mixes. Maintaining mixer cleanliness is crucial for efficacy and safety. Written maintenance schedules are necessary to prevent worn equipment parts from causing residue buildup during emptying. Mixers should operate for a preset time established through pre-production trials to ensure homogenous mixes. Regular checks of mixing efficiency are required to confirm the even dispersion of additives. This involves selecting a tracer, incorporating it into a mix, sampling, analyzing the tracer in samples, and evaluating the results achieved.
4.5 Product, Packaging & Process Design
During the design and development phase of pet food, packaging, and processes, a hazard analysis study (e.g., HACCP or HARPC) must be employed to identify and assess potential safety hazards. The pet food should be designed to ensure safety and meet its intended nutritional requirements. The manufacturer should verify that the manufacturing process can produce nutritionally appropriate, safe, and legal pet food through appropriate testing. Establishing shelf life, considering formulation, production, packaging, and storage conditions, is necessary, with records of shelf life assessments maintained. Shelf life should be validated based on criteria like microbiological, chemical, nutritional, and sensory analysis. Additionally, the packaging, process, and materials used should assure pet food safety.
4.6 Product Information & Consumer Awareness
Pet food products must have clear information about their content and intended use, usually provided on the product label. Procedures must be established to ensure proper labeling in accordance with relevant regulations. Information for industry or trade users should be distinguishable from consumer information on food labels. Consumers should also be educated about basic food hygiene standards to understand the importance of product information, make informed choices, and prevent contamination or foodborne pathogen growth. Products should include adequate information for the next person in the food chain to handle, store, and prepare them safely. Inadequate product information can lead to mishandling, potentially resulting in illness or unsuitability for consumption despite earlier hygiene control measures.
4.7 Rework
Rework should be regarded as a raw material, and consequently, fundamental principles must be observed. When rework or any reworking process occurs, traceability must be upheld. In cases involving re-processing or reworking operations, procedures should be put in place to guarantee the safety, legality, and quality of the final product. The incorporation of rework must be a part of the HACCP study, involving elements like inclusion in the flow diagram and consideration of potential hazards regarding contamination of the finished product.
4.8 Traceability and Recall Processes
Traceability is the ability to track feed materials, additives, packaging material, or any substances used at all stages of production, processing, and distribution of pet food. Pet food manufacturers need to be registered and approved by relevant authorities and maintain up-to-date records of their facilities. A robust traceability system with unique batch numbers is essential for identifying materials and products. This system must be regularly reviewed, tested, and documented to ensure it meets its objectives. Manufacturers should have tools in place for swift product recall and designate a Recall Coordinator and Team to manage recall procedures, safety investigations, communication, product disposition, and recall effectiveness verification.
A Digital Approach to Establish Pet Food Safety Through Smart Food Safe’s Software Solutions
In today’s fast-paced and ever-changing pet food industry, Smart Food Safe brings forward a consolidated suite of digital modules designed to revolutionize pet food safety and meet the stringent demands of this sector. These solutions are tailored to streamline pet food supply chain operations, improve compliance, and enhance food safety across the board.
Smart Docs simplifies the organization and management of critical documents, ensuring that pet food manufacturers have easy access to vital quality and safety documents, leading to quicker decision-making and improved compliance.
Smart Record offers a seamless transition from paper-based records to digital record-keeping to improve data accuracy by minimizing manual errors and enhancing traceability.
Smart Supplier helps in assessing, approving, and monitoring suppliers to minimize risks and maintain high-quality standards.
Creating and managing product specifications and recipes is made easy with Smart Specification, ensuring consistency and precision in pet food production.
Automation of product release and compliance management with Smart Compliance reduces the risk of errors, making it easier for pet food manufacturers to comply with regulatory standards.
Ensure that your workforce is well-informed and up-to-date with Smart Training , which provides a digital learning management platform for training on food safety and quality standards.
Smart Audit organizes the process of identifying gaps in food safety and quality systems through effective audits, followed by the implementation of required corrective and preventive actions.
Smart EMP automates environmental monitoring to minimize the opportunities for contamination in pet foods by maintaining a safe and hygienic environment.
Smart HACCP simplifies the creation of Hazard Analysis and Critical Control Points (HACCP) plans, ensuring that potential hazards are detected and controlled.
Ensure the quality of pet food ingredients and products with Smart Lab, a comprehensive LIMS software that manages laboratory data efficiently.
Smart Label ensures that pet food labels comply with regulations, displaying accurate nutritional facts and ingredient information.
In the unfortunate event of a product recall, Smart Recall aids in the swift and efficient management of the process to minimize harm and damage to your brand.
Quality and Food Safety Management Software
Food Safety and Quality Management Software to streamline processes, track compliance, ensure traceability and maintain audit readiness with global quality and food safety standards
A food safety incident that occurred in December 2020, causing the deaths of hundreds of pets due to contaminated pet food, sent shockwaves across the pet food industry and pet owners all over the globe. It resulted in the recall of about 60 million pounds of corn-based dog and cat food containing deadly levels of aflatoxins, despite the FDA’s maximum permissible limit of aflatoxin in pet food to be 20 parts per billion.
These unfortunate deaths could have been prevented if efficient safety management systems had been in place to control the food safety risks associated with the ingredients of pet foods.
All ingredients and processes used in the creation of pet food and treats are potential hazards, as rightly said by Gary Huddleston, Director of Feed Manufacturing and Regulatory Affairs, American Feed Industry Association (AFIA), in the article published in the July 2023 issue of Pet Food Processing. This is why pet food manufacturers need to appropriately monitor and regulate all elements of the ingredient life cycle to safeguard the safety of their pet products.
What is Pet Food?
Any product produced by a pet food manufacturer, whether processed, partially processed, or unprocessed, intended to be ingested by pet animals after being placed on the market. It is any commercial product formulated to provide essential nutrients and energy for domesticated animals and to meet their dietary requirements, considering factors like age, size, breed, and activity level. Pet food typically includes a combination of proteins, carbohydrates, fats, vitamins, and minerals, all tailored to support the pet’s health and growth.
Pet food is more than just a meal; it is a source of nutrition and sustenance for pet animals. To maintain proper bodily functions and ensure a pet’s well-being, it is crucial to be mindful of the ingredients in their food as well as ascertain optimal levels of safety management throughout the pet food production and processing till they reach the pets.
Why is it Important & Challenging to Ensure Food Safety in Pet Foods?
Ensuring food safety in pet foods is crucial for several reasons, as it comes with unique challenges such as:
Lack of Communication: Unlike humans, pets cannot verbally express their feelings or discomfort when they experience food-related problems. This makes it tedious to identify and trace back the source of foodborne illnesses or issues. In the case of human food safety incidents, if someone falls ill, they can describe what they ate, where they ate it, and when they consumed it. In contrast, pets cannot provide this information, making it difficult to pinpoint the exact cause of their illness.
Inconsistent Dietary Habits: Pets often have varied and unpredictable eating habits. They might graze throughout the day, eat from different sources, or consume various types of foods. This inconsistency in their dietary patterns further complicates the process of identifying the source of foodborne illnesses.
Limited Awareness and Understanding: Animals lack awareness and understanding of what they are eating. They do not have the ability to make choices based on food safety and quality considerations. This means that even if a pet food product is contaminated or of low quality, pets will not be able to avoid it. They rely on their owners or caregivers to make safe and healthy food choices for them.
Delayed Symptoms: In some cases, symptoms of foodborne diseases in pets may not appear immediately. It might take days or even weeks for signs of illness to manifest. This delayed onset of symptoms can make it even more problematic to link the illness to a specific food source.
Understanding Pet Food Ingredients
Pet food ingredients can be derived from either animals or plants. In a study conducted in 2020, it was discovered that pet food recipes utilize over 500 different ingredients, amounting to a total of more than 8.5 million tons of both animal- and plant-based products.
Many animal-derived ingredients used in pet food come from parts of the animal that are not consumed by humans due to cultural preferences and eating habits. These parts, such as lungs, hearts, kidneys, or liver, are perfectly safe for consumption once they have undergone veterinary inspections and been deemed suitable to consume.
On the other hand, ingredients of plant origin are often shared between human and pet nutrition, including items like maize, rice, wheat, and oats. Some ingredients are unique to pet food, as manufacturers seek particular combinations of nutritional properties, particularly beneficial for pets. These may include vegetable fibers to promote healthy digestion and pre-and probiotics to support digestive health, among others.
Maintaining Ingredient Safety by Controlling Hazards in Pet Foods
Each ingredient included in a pet food recipe and listed on the label should be carefully chosen by pet food manufacturers to serve a function for the pet. There are various types of hazards that can be present in pet food ingredients, according to the FDA’s Guidance for Industry, “Hazard Analysis and Risk-Based Preventive Controls For Animals,” each of which should be thoroughly supervised.
Known or Reasonably Foreseeable Hazards
Pet food can become contaminated with biological, chemical (including radiological), or physical hazards, depending on the type of pet food being manufactured, processed, packed, or held at your facility.
- Biological Hazards
Prominent biological hazards are bacterial pathogens (Salmonella spp., L. monocytogenes, and pathogenic E. coli), viral pathogens, and certain parasites (Toxoplasma gondii, Cryptosporidium spp.) that may contaminate pet food manufacturing operations. These pathogens can be:
i) Ingredient-related hazards – i.e., introduced from raw materials and other ingredients
ii) Process-related hazards – e.g., if the pathogens:
- survive the manufacturing process
- increase in number due to lack of time/temperature control or due to the animal food’s formulation
- are introduced into a finished animal food due to loss of container integrity
iii) Facility-related hazards – e.g., if the pathogens are introduced from:
- unsanitary animal food processing equipment
- cross-contamination between raw and cooked products
- contaminated air
- sewage or contaminated water
- Chemical Hazards
Chemical hazards include that are natural components of ingredients (e.g., glucosinolates) or natural toxins (e.g., mycotoxins), contaminants of raw materials and other ingredients (e.g., pesticides and drug residues), and hazards as a result of manufacturing errors (e.g., nutrient deficiencies or toxicities). These chemical hazards can be:
i) Ingredient-related hazards – i.e., introduced from raw materials and other ingredients such as:
- pesticide residues and mycotoxins on raw agricultural
- commodities and grains
- heavy metals in or on raw agricultural commodities or in mineral
- ingredients or premixes
- natural toxins (e.g., glucosinolates in the Brassicaceae family)
- animal drug residues
- unapproved food or color additives
- dioxins
ii) Process-related hazards – e.g., from manufacturing errors or cross-contamination such as:
- nutrient deficiencies or toxicities due to manufacturing error
- animal drug carryover from medicated to non-medicated animal
- food
- food or color additives not approved for certain species due to
- incomplete cleanout of equipment
iii) Facility-related hazards – e.g., from chemicals used on animal food processing equipment or utensils or chemicals stored in the facility such as:
- contamination with industrial chemicals such as cleaners or sanitizers
- chemicals not used in processing animal food but stored in a facility like fertilizers
- heavy metals due to leaching from containers or utensils
- Physical Hazards
Physical hazards are broadly classified as sharp hazards, choking hazards, and conditions of
animal food hazards such as size and hardness. These include:
- Metal (Ferrous and Non-Ferrous): Metal-to-metal contact during processing can introduce metal fragments into products. For example, metal fragments can break off during mechanical cutting and blending operations, and some metal equipment has parts that can break or fall off, such as wire-mesh belts. Metal screens may become worn over time or be torn, introducing metal fragments
- Glass: Glass fragments in animal food can cause injury to the animal eating the food. Most animal food facilities do not use glass containers for their animal food, but fragments may be introduced through the environment (e.g., overhead light fixtures made of glass that can fracture) or through ingredients that are contaminated with glass
- Hard Plastic: Hard plastic can be introduced into animal food when tools and equipment such as scoops, paddles, buckets, or other containers develop fatigue, crack, and break as they wear. It can also be introduced into animal food when plastic sieves and screens deteriorate.
- Conditions of Animal Food: It refers to the physical, mechanical, and other characteristics (e.g., particle size, hardness, surface roughness, digestibility, and ability to soften when moistened) of animal food that can cause injuries or illness in animals. Hazards related to the conditions of the animal food can occur when the particle size is too large to eat, resulting in starvation (e.g., crumbles too large for small birds) or alternatively, when the food is ground too fine as it can aerosolize and cause respiratory problems and corneal injuries. Also, a large portion of fines (i.e., very small particles from the milling or pelleting process) can lead to rapid fermentation by gut microflora, resulting in bloat in ruminants (e.g., cattle). Lack of digestibility can result in an obstructed digestive tract.
- Radiological Hazards
Contamination of pet food by radionuclides (a radiological hazard) is a rare event. The most common way these radionuclides are incorporated into animal food is through the use of water that contains the radionuclides. This water may be an ingredient in animal food or used during the manufacturing process, such as for washing ingredients or equipment. They may also result from accidental contamination, e.g., contamination arising from accidental release from a nuclear facility or from damage to a nuclear facility from a natural disaster.
Securing Safety Measures Throughout Ingredient Lifecycle: The Cornerstone for Safe Pet Foods
The pet food industry is witnessing a substantial transformation driven by several key factors. First, the increasing pet population has expanded the market for pet food, with more households adopting pets. Concurrently, pet owners are changing their preferences, seeking high-quality, safe, and natural ingredients for their furry family members. There’s a growing emphasis on pet health and wellness, driving the demand for pet food that contributes to overall well-being and supports specific health needs.
Furthermore, businesses that can provide sustainable, tailored, and transparent pet food options are well-positioned in this advancing landscape. Customized nutrition based on factors like age, breed, and health condition is gaining popularity, and pet owners increasingly value ingredient traceability and ethical sourcing. Social media also plays a pivotal role in influencing pet owner preferences and trends, making it essential for businesses to engage with pet owners through these platforms, showcase their commitment to quality and sustainability, and stay responsive to changing demands. This evolving market offers significant opportunities for companies that can align with these pet owner preferences and the broader shift towards more responsible and individualized pet food choices.
An overview of the key aspects of securing pet food safety in the journey of ingredients to your pet’s bowl in reference to the FEDIAF Guide To Good Practice for the Manufacture of Safe Pet Foods and GAPFA Global Pet Food Safety Guidance is given below.
Pet Food Safety Management System
The national and regional pet food industry associations and companies of pet food manufacturers across the globe establish their own guidelines to set up pet food safety management systems based on internationally recognized standards, e.g., EN-ISO 9000:2005 series, ISO 22000:2005 (or FSSC 22000), PAS 222, and Global Food Safety Initiative benchmark requirements.
A pet food safety management system should encompass several key components. It must establish a documented system that outlines the policy objectives of the pet food establishment, ensuring the production of safe and suitable pet food products. It should describe the organizational structure, defining roles and responsibilities related to product safety, as well as the allocation of resources and personnel training.
The system should include procedures for all stages of production, storage, and distribution while relying on records to validate and verify the safety and quality of pet food products. There should be detailed operational hygiene and process controls to maintain a clean and safe production environment. Furthermore, it should incorporate a Food Safety Risk Assessment plan for each product family produced, such as HACCP or HARPC along with internal audits and management reviews, with documented results and corresponding preventive/corrective actions. Finally, it must include a system for handling raw materials and packaging materials, including traceability to ensure product quality and safety. This system should be reviewed regularly for continuous improvement on at least an annual basis or if there is any change to the food safety system.
Let us delve into the fundamental elements of establishing a pet food safety management system:
1. Management Commitment
The management, spanning from higher to lower levels, must be dedicated to implementing pet food safety practices, including establishing a safety policy, ensuring regulatory compliance and good manufacturing practices, defining the scope of the safety management system, identifying potential risks to pet food production, and having a crisis management plan in place. Management-appointed staff should have clear responsibilities and authority to identify and document safety issues and initiate preventive and corrective actions to address product safety concerns.
1.1 Management Responsibility
The senior site management of a pet manufacturing facility mandates the creation and communication of a food safety policy, the establishment of a food safety culture, and the implementation of a food safety management system that complies with regulations and customer requirements. They must lead in setting food safety objectives, allocating resources, and fostering accountability among staff. It also necessitates the designation of responsible individuals, including backup personnel, for various roles within the food safety management system. Adequate staffing and alignment of departments to meet food safety goals are emphasized. Training and competencies of site personnel are to be ascertained, and the integrity of the food safety system must be maintained during organizational or personnel changes.
1.2 Management Review
The pet food safety management system requires an annual review by management, including updates to system documentation, assessment of food safety culture performance, evaluation of food safety objectives and performance measures, analysis of corrective and preventative actions, trends from audits, customer complaints, and verification activities, examination of the hazard and risk management system, and the review of follow-up actions from previous management assessments. Detailed records of all management reviews and updates must be retained for documentation purposes.
1.3 Complaint Management
The procedure for addressing food safety complaints related to pet food produced or handled on-site, consisting of the documentation and implementation of a process to handle complaints from commercial customers, consumers, and authorities, must be in place. Adverse trends in customer complaints must be investigated, with a focus on determining the root causes by knowledgeable personnel. Appropriate corrective and preventative actions should then be taken based on the severity of the incident and the root cause analysis. Records of customer complaints, their investigation, and the resolution process must be maintained for documentation and accountability purposes.
2. Pre-requisite Programmes
The following prerequisite programs outline the general pet food safety principles across the food supply chain:
2.1 Facilities & Plant Design
The site must be located and maintained to prevent contamination and should have measures in place to ensure ongoing effectiveness. External storage should protect items from contamination, and drainage should prevent standing water. The surrounding areas should be clean, well-kept, and pest-controlled. Waste collection areas should be clearly defined and managed to prevent contamination. Access to the site needs to be controlled to address security concerns.
Internally, the construction of the site should suit its purpose. The production process should allow for proper cleaning and disinfection to prevent contamination and cross-contamination. Sufficient working and storage space is needed. Segregation of materials and waste is essential to minimize cross-contamination, and facilities for disposing of unused animal byproducts should be available.
In terms of construction, walls, floors, and ceilings should facilitate cleaning and maintenance. Floors should have proper drainage. Ceilings and fixtures should prevent dirt, condensation, mold, and dust accumulation. Windows for ventilation should be screened to prevent pests. External openings should be managed to prevent foreign matter, moisture, and pests. Ample ventilation is required in product storage and processing areas. Finally, personnel hygiene facilities should be available and clearly designated, separate from production areas.
2.2 Utilities
The design and layout of utilities must take into account the prevention of product contamination. Whenever applicable, utility units must be placed away from walls to allow easy access for operation, cleaning, and maintenance and to prevent pest infestation. Utilities include:
Supply of water or steam as product ingredient or for product contact;
Supply of air (or gas) as ventilation (natural or mechanical) or as a compressed air or gas system;
Supply of energy (for example, electricity, light, or heat);
2.3 Waste Disposal
Effective waste management is important to prevent contamination in production areas. Waste collection, removal, and disposal systems should be in place. Waste containers must be clearly marked, positioned in designated areas, designed for easy cleaning and emptying, used exclusively for waste, and kept closed. Drains should be constructed and located to prevent material or product contamination, with adequate capacity to handle expected flow loads. Drainage should not carry contaminants from dirty to clean areas. Maintaining cleanliness and controlling spoilage and dust is essential to deter pests.
2.4 Equipment
Equipment in pet food production should be designed and placed to prevent contamination and enable effective cleaning and disinfection. Machinery that contacts feed materials or pet food should be dried after wet cleaning. Proper specification and commissioning of equipment, along with regular maintenance following the manufacturer’s procedures, are mandatory to maintain equipment. All equipment surfaces in contact with the product should be non-reactive and impermeable. Equipment used to monitor critical control points for Product Safety, Operational Prerequisite Programs, and product legality should be calibrated and traceable.
2.5 Cleaning & Sanitation
Maintaining hygienic conditions in pet food production requires establishing standards of hygiene and housekeeping. Only approved feed-grade cleaning and sanitizing agents should be used. Cleaning tools and equipment must be well-maintained to prevent potential sources of contamination. Cleaning and disinfection programs should be documented and cover the entire facility and equipment. These programs must be validated and verified for their effectiveness in reducing contamination risk, with periodic cleaning and sanitizing activities recorded.
2.6 Personnel
Personnel personal hygiene policies must be established, documented, and enforced. Staff involved in various aspects of production should be trained on these policies and other relevant procedures for product safety. The policies should cover hand washing, rules for eating, drinking, and smoking, clothing requirements, jewelry and watch usage, treatment of injuries, and staff access to post-kill-step product areas. Staff should flow between different areas to prevent cross-contamination, and facilities should be kept clean. Storage for personal effects and protective clothing, where necessary, should be provided, with separate eating and smoking areas away from production facilities. Visitors and contractors at the plant should adhere to the same personal hygiene procedures.
Training requirements for all staff, including temporary personnel and contractors, should be identified and documented. This includes induction training specific to the facility, covering product safety, personal hygiene, and relevant prerequisites like GMPs. Specific training needs, such as in food safety risk assessment systems (e.g., HACCP), and internal auditing, should be identified and scheduled. Records of training activities, competency assessments, and certificates should be maintained. The training program’s effectiveness should be periodically assessed.
2.7 Integrated Pest Management System
The pet food manufacturer is responsible for implementing procedures to minimize the risk of pest infestation, including hygiene, cleaning, materials inspection, and storage monitoring. Pest control programs should be in place and regularly reviewed for effectiveness. The manufacturer can either contract a competent and licensed pest control organization or have trained personnel for premises inspection and treatment. Electric fly killers and pest traps, if used, should be positioned to avoid product contamination. Detailed documentation on bait use and pest control measures’ locations on a site plan is necessary, along with keeping records of inspections, recommendations, and actions taken to address pest issues.
2.8 Foreign Bodies Control Policy
The use of glass or other brittle material (such as hard plastic components in equipment) close to production machinery must be avoided, and wherever necessary, it must be protected against breakage. Implement a zoning plan as identified in hazard assessments. Have written procedures in place for handling wood, metal, glass, and hard clear plastic breakages in various areas of the production process to ensure necessary precautions. These procedures should be part of a formal foreign body control policy. Establish measures to prevent, control, or detect potential contaminants identified in hazard assessments.
3. HACCP System
The team responsible for a pet food facility should establish a system that assesses potential food-related hazards in the manufacturing, processing, packaging, or storage of pet food. This system should identify and put into practice preventive measures to significantly reduce or eliminate these hazards. It is essential to continually monitor the effectiveness of these controls and maintain routine records of this monitoring and validation.
The applied Food Safety Risk Assessment system, such as HACCP, HARPC, or an equivalent, should adhere to internationally accepted standards. To conduct a food safety risk assessment, it should follow the steps and principles outlined in the ‘Hazard Analysis and Critical Control Point (HACCP) system and guidelines for its application’ as detailed in the Annex to Codex Alimentarius ‘Recommended International Code of Practice – General Principles of Food Hygiene’ (CAC/RCP 1).
The HACCP system is carried out in 12 steps, which follow the seven principles described in the Codex Alimentarius based on a prior implementation of pre-requisite programs.
Food Safety Management Software
Boost your food business’s hygiene standards with Smart Food Safe’s tech-driven solutions—streamline 4C processes to yield optimal results, and ensure compliance effortlessly.

| Principle | Description | Steps |
| Codex Principle 1 | Conduct a hazard analysis | Step 1: Assemble HACCP Team Step 2: Describe product Step 3: Identify intended use Step 4: Construct flow diagram Step 5: Confirm flow diagram on site Step 6: List all potential hazards Step 6: Conduct a hazard analysis Step 6: Consider control measures |
| Codex Principle 2 | Determine the Critical Control Points (CCPs) and Operational Pre- Requisite Programmes (OPRPs) | Step 7: Use decision tree to determine CCPs and OPRP |
| Codex Principle 3 | Establish critical limits | Step 8: Establish action and critical limits for each CCP and OPRP |
| Codex Principle 4 | Establish CCP monitoring procedures | Step 9: Establish monitoring systems for each CCP and OPRP |
| Codex Principle 5 | Establish corrective action plans | Step 10: Establish the corrective action to be taken when monitoring indicates that a particular CCP or OPRP is not under control |
| Codex Principle 6 | Establish verification procedures | Step 11: Establish procedures for verification to confirm that the HACCP system is working effectively |
| Codex Principle 7 | Establish documentation and record keeping systems | Step 12: Establish documentation concerning all procedures and records appropriate to these principles and their application |
4. Production Practices in Pet Manufacturing
The production practices in pet manufacturing should be such that the manufacturing environment and personnel should not be a source of risk to product safety.
4.1 Management of Incoming Materials
Incoming feed materials, additives, and packaging materials must meet safety and legal standards. Procedures should cover registration, inspection, analysis, acceptance, and rejection of these materials, including how to handle and track rejected deliveries.
Vendor/Supplier Approval & Assessment System: A system for approving vendors and suppliers is paramount for sourcing safe feed materials. It includes site approval, purchase control, and documentation of standards and monitoring procedures for primary production, inbound materials, and packaging. Approved supplier lists should be maintained, and assessment may involve continuous monitoring, inspection, traceability, HACCP system evaluation, product safety, and legislative compliance.
Specifications: Written specifications must define safety and legal compliance for feed materials, additives, and packaging materials and be regularly updated. Each material should have a specification agreed upon with the relevant parties. Specifications reflect risk analysis for physical, chemical, and biological risks per HACCP. Procedures should exist for specification amendment and approval across the process.
Handling of Incoming Materials: Inspect vehicles, documentation, and materials before unloading to confirm correctness and suitability. Check incoming materials against specifications using Certificates of Analysis (COA). Document procedures for inspection, sampling, and analysis, including information on products, hazards, methods, frequency, qualifications, and responsibilities. Establish procedures for dealing with non-conforming materials and document rejected shipments. Maintain documentation of rejected shipments, including reasons for rejection. Ensure perishable or frozen materials meet minimum temperature requirements during shipment, transportation, and receipt, and document temperature checks at receiving points for perishable goods.
4.2 Pathogens Monitoring
When a pet food safety risk linked to pathogens is defined in the HACCP study, a monitoring program should be implemented in order to proactively control the safety of products. Pathogen monitoring is a way to ensure the safety of pet food and is one of the programs aimed at measuring the effectiveness of the implementation of the pre-requisite programs, in particular for products that are not sterilized in hermetic packages. Zoning and a pre-defined flow of products and personnel is an important prerequisite program to proactively prevent pathogen presence.
In order to monitor the microbiological state of a factory, samples are collected and analyzed according to a pre-defined plan (location, frequency, number). Data obtained from the monitoring should be routinely trended, and the information obtained will drive subsequent sampling and product release protocols as well as corrective actions. The type of pathogen (and indicator) microorganisms to be monitored will be defined by the hazard analysis (HACCP plan). Pathogen monitoring control programs must be implemented, including environmental samples, line samples, and finished product samples, and should be subject to regular review.
4.3 Transport & Storage
All vehicles and warehouses used for storing or transporting ingredients and products must be suitable for their intended purpose and maintained in a hygienic condition. Ingredient usage and shipping schedules should follow a FIFO or FEFO strategy where possible. Storage areas should be designed to prevent damage, contamination, mixing, or deterioration. Clear identification of storage areas is necessary, and regular inspections for structural integrity and content conditions, especially in dry ingredients, are vital. Adequate ventilation and capacity for chilled and frozen products are required. Storage areas should be kept clean and free from contamination risks. Procedures to prevent cross-contamination of finished products, packaging, and ingredients should be in place, and processed pet food and packaging material must be separated from unprocessed ingredients to avoid cross-contamination.
4.4 Mixing & Homogeneity
Mixers used in pet food manufacturing should be suitable for the range of weights or volumes they handle and capable of producing homogeneous mixes. Maintaining mixer cleanliness is crucial for efficacy and safety. Written maintenance schedules are necessary to prevent worn equipment parts from causing residue buildup during emptying. Mixers should operate for a preset time established through pre-production trials to ensure homogenous mixes. Regular checks of mixing efficiency are required to confirm the even dispersion of additives. This involves selecting a tracer, incorporating it into a mix, sampling, analyzing the tracer in samples, and evaluating the results achieved.
4.5 Product, Packaging & Process Design
During the design and development phase of pet food, packaging, and processes, a hazard analysis study (e.g., HACCP or HARPC) must be employed to identify and assess potential safety hazards. The pet food should be designed to ensure safety and meet its intended nutritional requirements. The manufacturer should verify that the manufacturing process can produce nutritionally appropriate, safe, and legal pet food through appropriate testing. Establishing shelf life, considering formulation, production, packaging, and storage conditions, is necessary, with records of shelf life assessments maintained. Shelf life should be validated based on criteria like microbiological, chemical, nutritional, and sensory analysis. Additionally, the packaging, process, and materials used should assure pet food safety.
4.6 Product Information & Consumer Awareness
Pet food products must have clear information about their content and intended use, usually provided on the product label. Procedures must be established to ensure proper labeling in accordance with relevant regulations. Information for industry or trade users should be distinguishable from consumer information on food labels. Consumers should also be educated about basic food hygiene standards to understand the importance of product information, make informed choices, and prevent contamination or foodborne pathogen growth. Products should include adequate information for the next person in the food chain to handle, store, and prepare them safely. Inadequate product information can lead to mishandling, potentially resulting in illness or unsuitability for consumption despite earlier hygiene control measures.
4.7 Rework
Rework should be regarded as a raw material, and consequently, fundamental principles must be observed. When rework or any reworking process occurs, traceability must be upheld. In cases involving re-processing or reworking operations, procedures should be put in place to guarantee the safety, legality, and quality of the final product. The incorporation of rework must be a part of the HACCP study, involving elements like inclusion in the flow diagram and consideration of potential hazards regarding contamination of the finished product.
4.8 Traceability and Recall Processes
Traceability is the ability to track feed materials, additives, packaging material, or any substances used at all stages of production, processing, and distribution of pet food. Pet food manufacturers need to be registered and approved by relevant authorities and maintain up-to-date records of their facilities. A robust traceability system with unique batch numbers is essential for identifying materials and products. This system must be regularly reviewed, tested, and documented to ensure it meets its objectives. Manufacturers should have tools in place for swift product recall and designate a Recall Coordinator and Team to manage recall procedures, safety investigations, communication, product disposition, and recall effectiveness verification.
A Digital Approach to Establish Pet Food Safety Through Smart Food Safe’s Software Solutions
In today’s fast-paced and ever-changing pet food industry, Smart Food Safe brings forward a consolidated suite of digital modules designed to revolutionize pet food safety and meet the stringent demands of this sector. These solutions are tailored to streamline pet food supply chain operations, improve compliance, and enhance food safety across the board.
Smart Docs simplifies the organization and management of critical documents, ensuring that pet food manufacturers have easy access to vital quality and safety documents, leading to quicker decision-making and improved compliance.
Smart Record offers a seamless transition from paper-based records to digital record-keeping to improve data accuracy by minimizing manual errors and enhancing traceability.
Smart Supplier helps in assessing, approving, and monitoring suppliers to minimize risks and maintain high-quality standards.
Creating and managing product specifications and recipes is made easy with Smart Specification, ensuring consistency and precision in pet food production.
Automation of product release and compliance management with Smart Compliance reduces the risk of errors, making it easier for pet food manufacturers to comply with regulatory standards.
Ensure that your workforce is well-informed and up-to-date with Smart Training , which provides a digital learning management platform for training on food safety and quality standards.
Smart Audit organizes the process of identifying gaps in food safety and quality systems through effective audits, followed by the implementation of required corrective and preventive actions.
Smart EMP automates environmental monitoring to minimize the opportunities for contamination in pet foods by maintaining a safe and hygienic environment.
Smart HACCP simplifies the creation of Hazard Analysis and Critical Control Points (HACCP) plans, ensuring that potential hazards are detected and controlled.
Ensure the quality of pet food ingredients and products with Smart Lab, a comprehensive LIMS software that manages laboratory data efficiently.
Smart Label ensures that pet food labels comply with regulations, displaying accurate nutritional facts and ingredient information.
In the unfortunate event of a product recall, Smart Recall aids in the swift and efficient management of the process to minimize harm and damage to your brand.

Quality and Food Safety Management Software
Food Safety and Quality Management Software to streamline processes, track compliance, ensure traceability and maintain audit readiness with global quality and food safety standards


